Unlocking the Spectrum: A Leap in Fluorescent Labeling of Cell Surface Proteins
Biomedical research is an area that is always changing. Scientists are always trying to find new ways to look at and understand the cells that make up living things. Fluorescence imaging, a way for researchers to see where proteins are and how they interact with each other in real time, has been one of the most important steps forward in this study. Traditional ways of attaching fluorescent markers to proteins, on the other hand, have their flaws and often mess up the protein’s normal interactions and behavior.
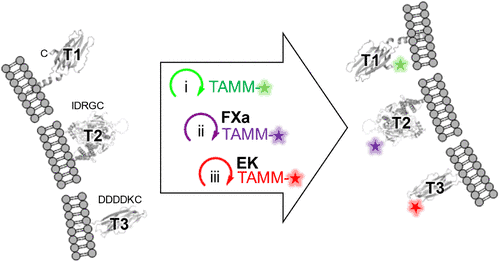
With their new research, a group of scientists from universities in China and the UK may change the way we do these fluorescent studies forever. A detailed study released by the American Chemical Society describes their newest discovery, which is a new chemical reaction called 2-[(alkylthio)(aryl)methylene]malononitrile (TAMM) condensation. Labeling cell surface proteins with this method is much easier and more effective, and it doesn’t change how they work. This makes it possible for more accurate biology research.
The Challenge of Conventional Methods
In the past, fluorescence imaging relied on joining proteins with fluorescent proteins or adding small molecules fluorophores using different tags or linkers. Even though these methods work, they have major problems. The big size of fluorescent proteins can make it harder for them to do their job and connect with other parts of cells. Using antibodies to deliver the fluorescent tags is another popular method, but it also has problems because the antibodies are big and need very specific conditions to bind.
A Smaller, Faster Alternative
Dr. Yi-Lin Wu from Cardiff University and Dr. Yu-Hsuan Tsai from the Shenzhen Bay Laboratory came up with the TAMM condensation method, which takes these problems straight on. This new method lets us study proteins in more detail without using extra protein tags or antibodies because it uses small-molecule fluorophores that bond to proteins through a quick and very selective chemical reaction.
Over 10,000 times per second, the TAMM molecules respond with proteins, which is a very fast rate. This happens in very mild conditions that protect the proteins’ delicate state. This speed is very important because it makes sure that the fluorescent tags can be attached quickly and correctly, before the cell goes through any changes that could change the protein’s normal state.
Expanding the Color Palette
One of the most important new developments in the study is that TAMM condensation can now be used with other labeling methods, such as tetrazine ligation and copper-catalyzed azide-alkyne cycloaddition (CuAAC). Because of this, researchers can label different proteins in the same cell using different colors. In real life, this means that scientists can now keep an eye on multiple processes at once and see how different proteins work and connect with each other in real time.
Revolutionizing Research and Beyond
Applications with live cells and in vivo
The best thing about TAMM condensation is that it can be used on live cells and even whole species because it is gentle and flexible. The study showed that labeling cell surface proteins worked well not only in cultured cells but also in live mice. This is a big step toward using this technology in the future for medical diagnostics and maybe even therapeutic interventions.
Changing the way research is done and more
This is a big step forward in bioconjugation chemistry, which is the study of how to connect biologically active molecules to each other or to other substances. Researchers can now watch cellular processes as they happen because protein labeling has been improved. This gives them a better picture of how cells work in health and sickness.
As scientists keep looking into and improving TAMM condensation, this technology has a huge chance to help medical science. Being able to correctly track many proteins at once has huge and exciting implications, ranging from helping us better understand how diseases work to creating new treatments that focus on specific parts of cells.
In a nutshell, Dr. Wu, Dr. Tsai, and their colleagues’ work not only improves what we can do with fluorescence imaging, but it also paves the way for future discoveries that could change the way we do biological and medical study.
Please see the article: A Single Bioorthogonal Reaction for Multiplex Cell Surface Protein Labeling
Yang Huang, Chengyang Wu, Anjing Lu, Jingzhe Wang, Jian Liang, Han Sun, Liqing Yang, Shixiang Duan, Andrey A. Berezin, Chuanliu Wu, Bo Zhang, Yi-Lin Wu, and Yu-Hsuan TsaiJournal of the American Chemical Society Article ASAP DOI: 10.1021/jacs.4c11701 https://pubs.acs.org/doi/10.1021/jacs.4c11701?articleRef=control